The South African Health Products Regulatory Authority (SAHPRA) together with the World Health Organisation (WHO) are introducing the pilot programme on the implementation of the National Action Plan (NAP) on the fight against substandard and falsified medical products (SFMPs). The NAP includes prevention, detection and response strategies.
This pilot programme is a year-long journey, involving SAHPRA and WHO working together with key stakeholders to the NAP in order to prevent, detect and respond to SFMPs in a united and collaborative manner.

The NAP will rely on the WHO handbook the implementation of the NAP for fighting SFMPs. The handbook frames the strategic objectives and goals, with actions, inputs, outputs and outcomes used for developing strategic and operational plans, and provides insights into approaches for operational planning, monitoring and evaluation.
A significant percentage of SF products circulating globally are found in Africa and this presents a serious concern for public health, affecting the attainment of Sustainable Development Goals (SDG) 3, which aims to ensure universal health access for all. A common finding from the WHO Global Benchmarking Tool assessments in various countries is the need to strengthen the market surveillance and control function – which fights the SFMPs.
The pilot begins in 2024 and is expected to be completed within 12 months.
Background
SAHPRA recently hosted a webinar that provided information on the project summary, and roles and highlighted the importance of stakeholder buy-in and active participation to ensure the country’s success in the fight against SFMP for this pilot project, and most importantly for the country and region. Subsequently, post-pilot, the WHO handbook on the implementation of the NAP for fighting SFMPs will be rolled out to all member states.
The NAP objectives are as follows:

provide countries with a framework and tools…
to develop national action plans to implement the Prevent, Detect and Respond.

provide guidance…
on how NRA’s can leverage the competencies of relevant stakeholders, including policymakers, procurers, distributors, practitioners, patients and consumers.

promote good governance and collaboration…
among the multiple stakeholders interested in the topic, they are responsible for regulatory control, surveillance, and oversight and participate in combating SF medical products.

support NRAS…
in achieving a stable, integrated and well-functioning regulatory system, particularly in specific aspects of market control, surveillance and regulatory inspections.
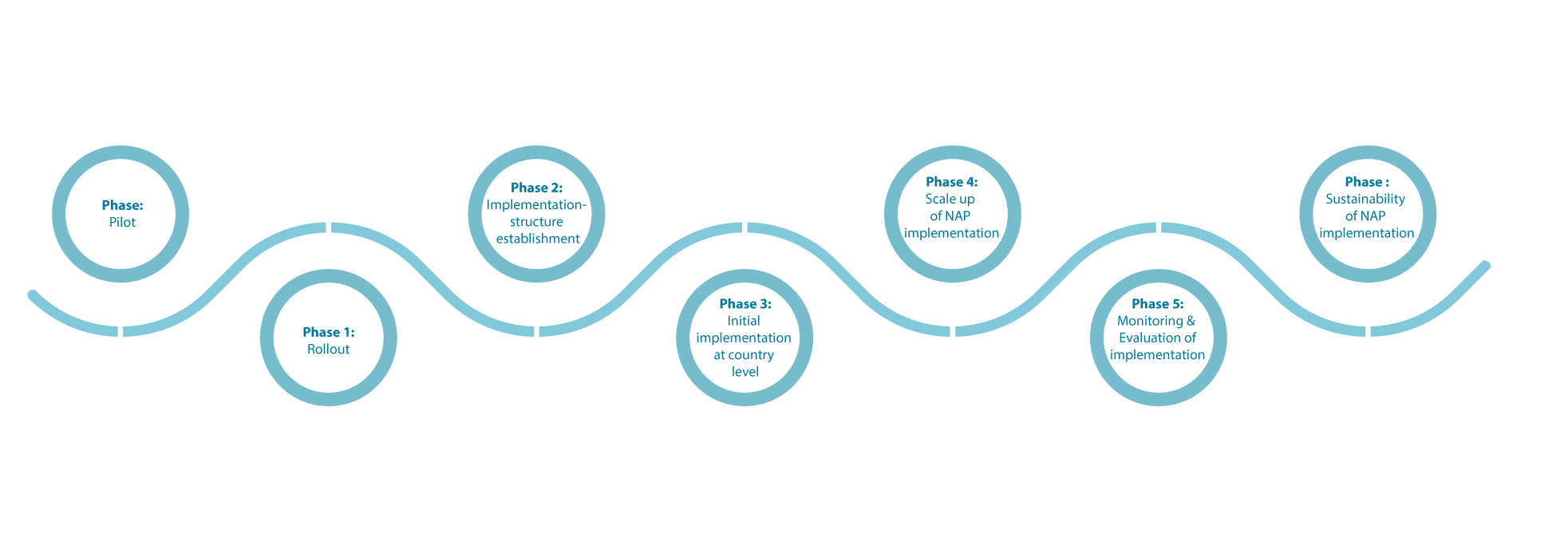
Implementation Roadmap
WHO Consultant for Project

Averouz Maritz
Medical Products Quality OfficerThe Medical Products Quality Officer (MPQO) consultant provides technical support for the development, implementation, monitoring and evaluation of the National Action Plan (NAP) on substandard and falsified (SF) medical products in South Africa. The MPQO works directly with the South African Health Products Regulatory Authority (SAHPRA) and national stakeholders to enhance the capacity of South Africa, through targeted support and alignment with the strategies for prevention, detection and response to SF medical products.
